
Songping Huang
Biography
Materials & Medicinal Chemistry
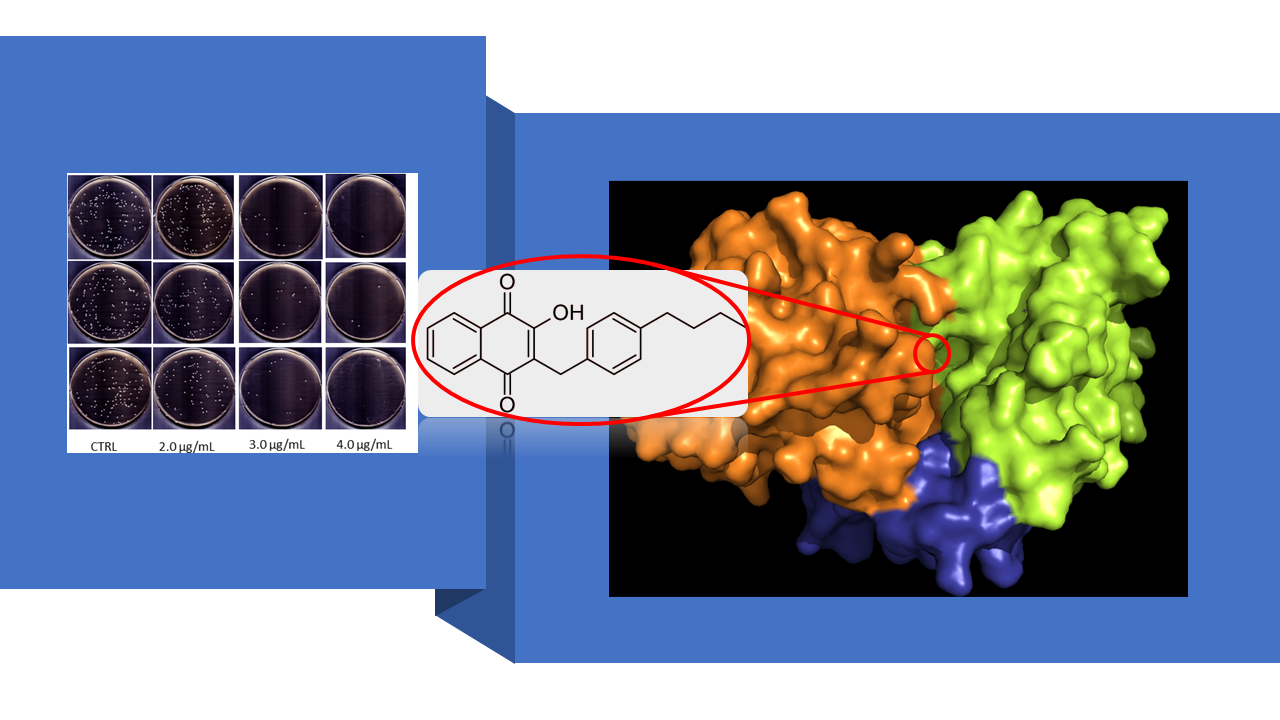
Research work in Huang group is divided into three interrelated areas: (1) rational design and synthesis of small-molecule antimicrobial agents that have different cellular or molecular targets than those of the conventional antibiotics for combating antimicrobial resistance (AMR). As an example of using this design principle to discover new molecules that can evade drug resistance development, we recently synthesized a Lawson derivative (6c) as an effective NDH-2 inhibitor. NDH-2, also known as type II dehydrogenase, is a peripheral membrane protein functioning as an enzyme (EC: 1.6.99.3) to catalyze the electron transfer in respiratory chain of certain pathogenic organisms e.g., Staphylococcus aureus (SA), but not expressed in mammals, hence constituting an ideal target for rational drug design. Since NDH-2 is not a known target of any existing antimicrobial drugs, 6c exhibits not only unusual potency against SA, but also the remarkable ability to overcome the drug resistance in a number of antibiotic resistant SA mutant bacteria. Currently, 6c is under preclinical animal studies for development as a topical antimicrobial drug to treat skin and soft-tissue MRSA (i.e., methicillin-resistant staphylococcus aureus) infections, particularly those by the MRSA mutant bacteria that are resistant to mupirocin and fusidic acid, the two widely used topical antibiotics for treating skin and soft-tissue MRSA infections.
(2) Exploration of ferroptosis - a special type of programmed cell death triggered by cellular dysregulation of iron, as the mode of action to develop Fe-based metallodrugs for cancer chemotherapy with the aim to improve the selectivity of cytotoxicity in cancerous cells vs. normal cells to reduce toxic side effects of anticancer drugs. Iron is known as a double-edged sword in biology: it is essential for almost all forms of life for their survival, division and growth, yet iron is potentially cytotoxic as dysregulated cellular iron uptake can result in reactive oxygen species (ROS)-triggered cell death from the iron-catalyzed Fenton reaction. Through the judicial selection of lipophilic iron chelators with high affinity for Fe(III) as the ionophores, we have demonstrated that certain octahedral Fe(III) complexes with D3 symmetry have the remarkable ability to transport iron across the cell membrane to trigger intracellular Fenton reaction, which affords potent broad-spectrum in vitro anticancer activity against a plethora of cancer cell lines including the Pt-resistant ovarian cancer cell line. Since the cellular targets and the mode of action of such Fe(III) complexes are drastically different and nonoverlapping with those of cisplatin, they have the distinct ability to overcome Pt resistance in A2780cis ovarian cancer cells.

Our work in this area raises the possibility of harnessing ferroptosis to develop Fe-based metallodrugs for cancer chemotherapy; and (3) development of nanoparticles of Prussian blue and its analogues as oral contrast agents (CAs) for diagnostic use in the gastrointestinal (GI) tract with magnetic resonance imaging (MRI) modality. The current commercial CAs use small-molecule Gd3+-chelates that do not have sufficient stability in acidic environment of the stomach and temporal stability when when they move through the GI tract, making the development of oral MRI contrast agents based on the platform of Gd3+-complexes problematic. Our group pioneered the use of biocompatible NPs of PB and its many analogues as oral contrast agents for MRI applications. Such NPs exhibit extremely high stability in the gastric juice, high r1 relaxivity, low toxicity and high temporal stability, showing great potential for the development of true T1-weighted oral contrast agents for MR imaging of the entire GI, an unmet clinical need in diagnostic medicine.

Scholarly, Creative & Professional Activities
- N. Abeydeera, B. Yu, P. D. Bishnu, M. Kim, S. D. Huang “Harnessing the Toxicity of Dysregulated Iron Uptake for Killing Staphylococcus aureus: Reality or Mirage?” Biomaterials Science (IF=6.843),10, 474-484 (2021). https://doi.org/10.1039/D1BM01743H
- Z. Wang, J. Li, B. M. Benin, B. Yu, S. D. Bunge, N. Abeydeera, S. D. Huang, M.-H. Kim “Lipophilic Ga Complex with Broad-Spectrum Antimicrobial Activity and the Ability to Overcome Gallium Resistance in both Pseudomonas aeruginosa and Staphylococcus aureus” Journal Medicinal Chemistry (IF=7.446), 64: 9381-9388 (2021). https://doi.org/10.1021/acs.jmedchem.1c00656
- M. S. Kandanapitiye, T. M. Dassanayake, A. C. Dassanayake, J. Shelestak, R. J. Clements, S. D. Huang “K2Mn3[FeII(CN)6]2 NPs with High T1-Relaxivity Attributable to Water Coordination on the Mn(II) Center for Gastrointestinal Tract MR Imaging” Advanced. Healthcare Materials (IF=9.933), 10(20): 2100987 (2021). https://doi.org/10.1002/adhm.202100987
- Dyne E, Prakash P, Li J, Yu B, Schmidt T, Huang S, Kim MH. “Mild Magnetic Nanoparticle Hyperthermia Promotes the Disaggregation and Microglia-mediated Clearance of Beta-Amyloid Plaques” Nanomedicine: Nanotechnology, Biology, and Medicine (IF=6.458), 34:102397 (2021). doi.org/10.1016/j.nano.2021.102397
- Song R, Yu B, Friedrich D, Li J, Shen H, Krautscheid H, Huang SD, Kim MH. “Naphthoquinone-derivative as a synthetic compound to overcome the antibiotic resistance of methicillin-resistant S. aureus” Communications Biology (IF=6.268), 3(1):529 (2020). https://www.nature.com/articles/s42003-020-01261-0
- Yu B., Wang Z., Almutairi L., Huang S., Kim MH “Harnessing iron oxide nanoparticles towards the improved bactericidal activity of macrophages against Staphylococcus aureus” Nanomedicine: Nanotechnology, Biology, and Medicine (IF=6.458), 24:102158 (2020). https://pubmed.ncbi.nlm.nih.gov/31982615 https://onlinelibrary.wiley.com/doi/abs/10.1002/ange.201713177
- Wang Z, Yu B, Alamri H, Yarabarla S, Kim MH, Huang SD “KCa(H2O)2[FeIII(CN)6].H2O nanoparticles as a novel antimicrobial agent for Staphylococcus aureus” Angewandte Chemie IntEd ((IF=15.334).) 37:2214-2218 (2018). https://onlinelibrary.wiley.com/doi/abs/10.1002/ange.201713177
