About COVID-19 Vaccines
About COVID-19 Vaccines
How does the vaccine work to protect you against COVID-19?
- Both Pfizer and Moderna are manufacturing a vaccine using messenger ribonucleic acid (mRNA). mRNA is naturally found in humans; its role is to deliver instructions from DNA to cells about which proteins the cell needs to create.
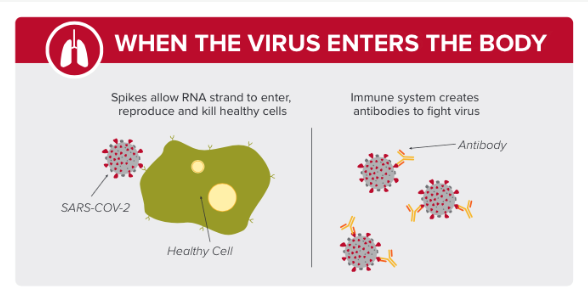
- The mRNA in the vaccine teaches the cell to make the virus’s spike protein – the main protein that is recognized by the body during the immune response. The body then produces antibodies against the virus’s spike protein. Those antibodies are then able to recognize the SARS-CoV-2 virus that causes COVID-19 if the recipient comes into contact with the virus and can more effectively clear the virus from the body and prevent severe infection.
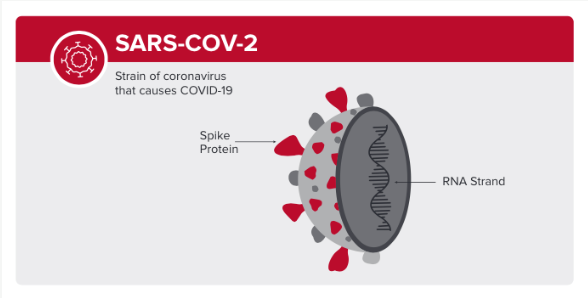 .
. 
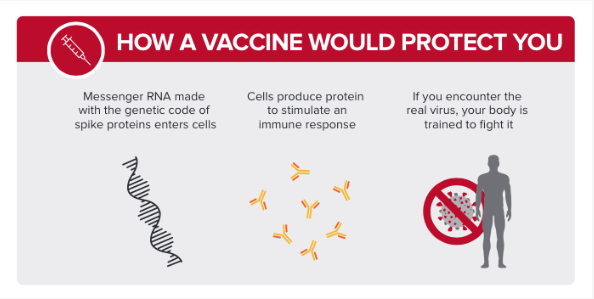
Did this vaccine go through a clinical trial?
- Yes. Operation Warp Speed is an effort to develop vaccines and therapeutics quicker than would normally be possible by running the three phases of these clinical trials in an accelerated time frame. The vaccine companies also start manufacturing on a large scale before the studies are done, betting that the vaccine will be effective. All the vaccines go through the normal steps, just in a much faster process, made possible, in part, by the large number of participants in each trial.
- This six-minute video and the illustration below further explain these points.
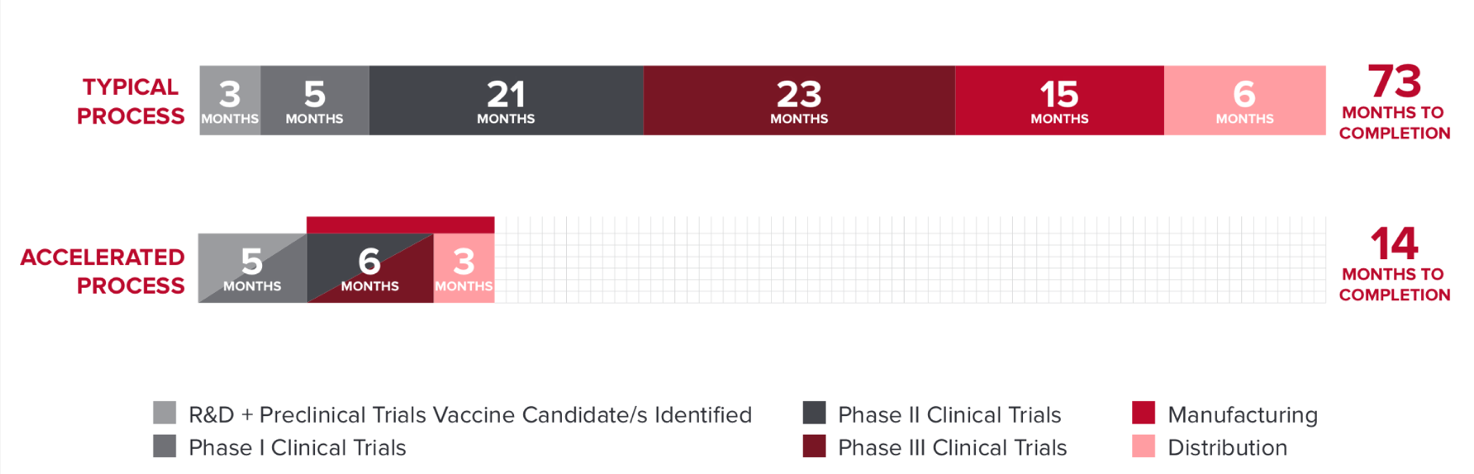
How many people received the vaccine during the trials?
- The Pfizer vaccine study enrolled 43,538 participants. The Moderna vaccine study enrolled 30,000 participants.
What is the efficacy of the vaccines being considered for emergency use?
- Both the Pfizer and Moderna vaccines were found to have an approximately 95% efficacy against developing serious COVID-19 disease after two doses were received.
- Johnson & Johnson vaccine was 66.3% effective in clinical trials.
What is an Emergency Use Authorization (EUA)?
- An EUA is a legal mechanism that allows the FDA to authorize the use of a medical product to address public health emergencies if certain statutory criteria and scientific evidence are met. This video provides a brief overview.
- The FDA will make publicly available all the data and information regarding EUA granted to COVID-19 drugs and vaccines.
Can I still get COVID-19 even if I am vaccinated?
- Possibly. The vaccine trials examined whether the vaccine prevented serious disease. You may be able to be infected and develop a more mild disease or an infection without symptoms, and you may be able to spread the virus. Additional studies are looking at both questions.
This vaccine was developed in record time. Is it safe?
- The vaccine is deemed to be safe based upon a rigorous evaluation of currently available scientific evidence. The most common reported adverse events were headaches, pain at the injection site, fatigue and a general feeling of unwellness.
Has the vaccine been approved by the Food and Drug Administration (FDA)?
- The vaccine is receiving Emergency Use Authorization (EUA) from the FDA. Under the EUA process, in emergency situations when there are no adequate, approved and available alternatives, the FDA has the authority to authorize medical products for use under specified conditions before all the evidence that would be needed for full FDA approval is available.
